It is our pleasure to announce that we will be attending MEDICA.
MEDICA is the world’s largest medical event. This year, it will be from the 12th – 15th of November, at Messe Düsseldorf; and attracts experts from all around the world: in the fields of business, research and politics. You will be able to attend multiple panels and discussions on the newest trends in the medical industry, see the newest technological innovations and meet with professionals from around the world.
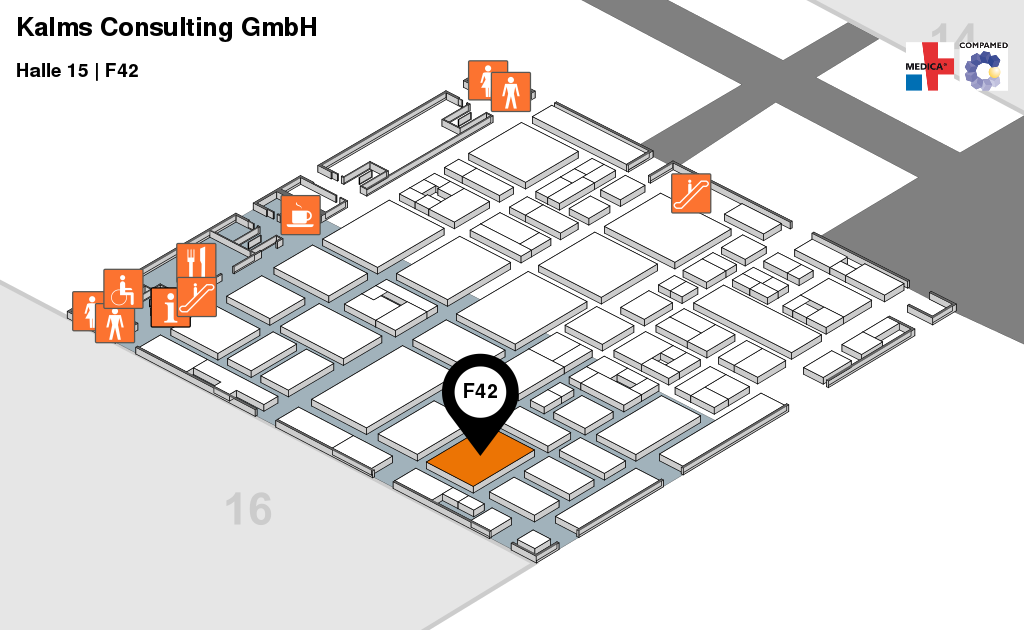
We would like to invite you to the Welcome Reception for the joint Berlin-Brandenburg booth. It will be at hall 15 booth F42 on Monday, November 12th, 2018, from 6:00pm-10:00pm. While we are at MEDICA, feel free to visit us at the Berlin-Brandenburg booth (hall 15 at booth F42).
We welcome your questions and topics that you would like to discuss with us. The whole Kalms Group (Kalms Consulting GmbH & Kalms Operations) will be present; giving you the opportunity to ask us about anything, from reimbursement and market access to logistics and warehousing.
We have also opened our appointment bookings service. Here you can setup an appointment to speak with renowned experts about commercialization in Europe, market access & reimbursement in the USA, market access in China and European medical device law. We are happy to have these experts at our booth and hope you will enjoy their knowledge and their distinguished experience as well.
We look forward to seeing you at MEDICA 2018!